Redox Potential Tuning
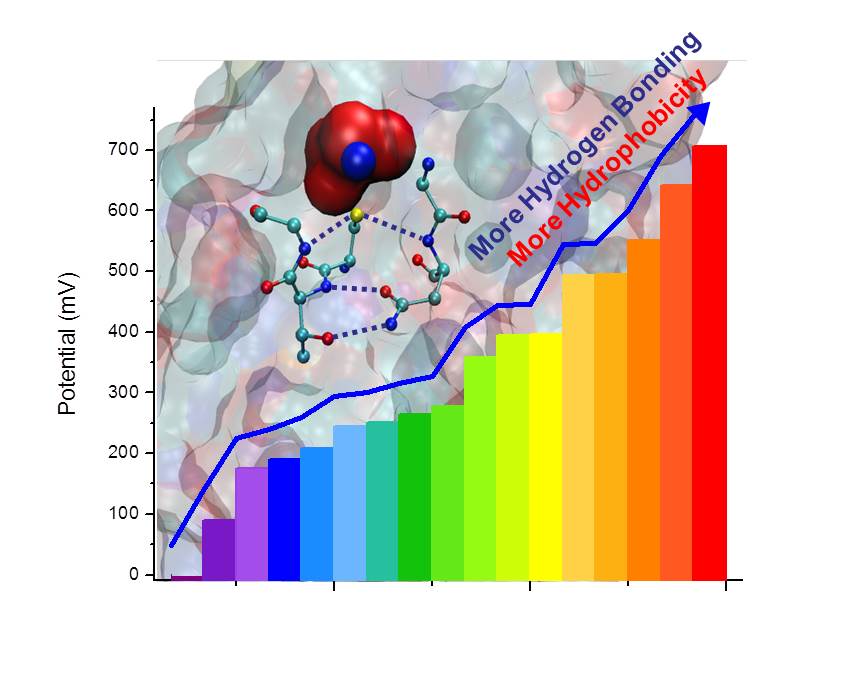
Redox potentials (Eº) are at the heart of many chemical and biological processes from electron transfer (ET) in photosynthesis and respiration to catalysis in water oxidation and N2 fixation. In order to properly function, the active sites in these processes require precisely tuned Eº. While it is well known that nature uses a limited set of primary coordination spheres to span a wide range of Eº, the factors that control the Eº, especially those that do not perturb the primary coordination spheres, remain elusive. Since the primary coordination sphere defines ET efficiency and catalytic reactivity of metalloproteins, the reactivity can only be tuned by altering secondary coordination sphere interactions to ensure a minimal effect on ET or catalytic efficiency.
To address this issue, we explore novel concepts through systematic tuning of the Eº of metal centers in our protein systems. Some examples of our methodology include:
- Rational design of the secondary coordination sphere interactions around the primary coordination spheres, resulting in a copper protein (azurin) that displays redox potentials beyond the natural potential range of the entire family of mononuclear copper redox proteins.
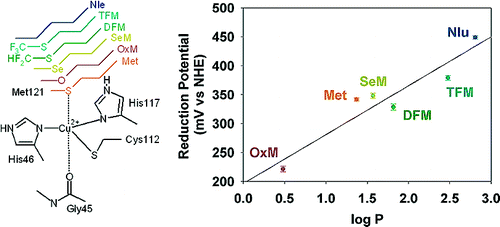
- Incorporation of unnatural amino acids, which has allowed us to use isostructural replacement to tune the redox potentials even further than using natural amino acids with minimal perturbation of the primary coordination spheres.
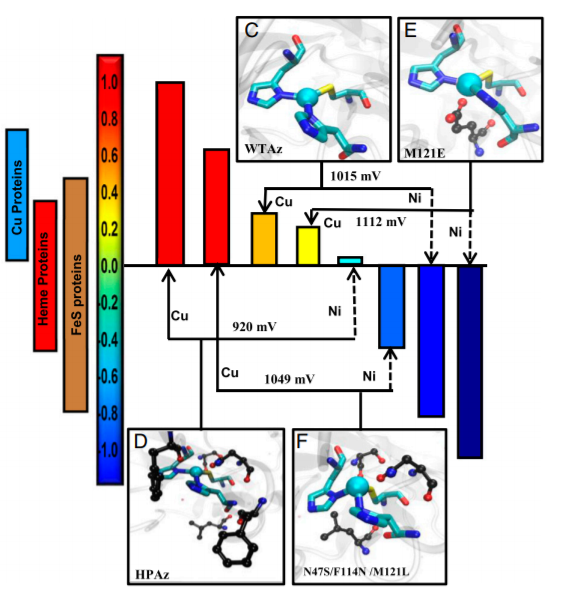
- A combination of secondary sphere tuning and metal substitutions, which has allowed us to tune the reduction potential of azurin to expand the entire physiological range of potentials from -1V to 1V vs. SHE.
These projects will allow us to advance fundamental knowledge of structural features for systematic control the metal centers’ Eº without perturbing their primary coordination spheres, as well as to demonstrate transformative potentials of such research in applying the knowledge to design:
- Water-soluble and stable redox agents with tunable Eº for biochemical studies; many of these redox agents are available to the wide scientific community through this web site.
- ET proteins with controlled Eº that can transfer electrons in the inverted region of Marcus theory ( Proc. Natl. Acad. Sci. USA 110, 10536-10540 (2013) ; J. Phy. Chem. Lett. 6, 100-105 (2015) ) for applications in photosynthesis and respiration.
- Biocatalysts with tunable activities through potential tuning ( J. Am. Chem. Soc. 136, 11882-11885 (2014) ).