Novel Sensing and Imaging to Advance Metallomics, Metabolomics and Glycomics in Biology
Selective sensors and imaging agents are very useful for on-site and real-time detection in environmental monitoring, food safety, medical diagnostics, and imaging. While much progress has been made in detecting large molecule targets, such as nucleic acids and proteins, sensing and imaging small molecule targets and biomarkers, such as metal ions and organic metabolites, remain difficult because they are subtle in structural differences and present in trace amounts. In addition, glycoRNAs have recently been discovered in 2021, but their spatiotemporal distributions are not known. Therefore, sensing and imaging in metallomics, metabolomics and glycomics have become a new frontier for chemical biologists following advancements made in genomics and proteomics. We have identified challenges in both fundamental science and in technological development, and we have made significant progress in meeting these challenges and successfully applied these sensors and imaging agents in understanding roles of lithium as a drug for bipolar disorder, roles of redox cycles of Fe2+/Fe3+ in Alzheimer diseases and roles of glycoRNAs in immunology and cancers.
For a general overview of this area, refer to the slideshow on the right and the following review articles:
Acc. Chem. Res. 52, 3275-3286 (2019), Inorg. Chem. 58, 13696-13708 (2019), Adv. Healthcare Mater. 8, 1801158 (2019), Curr. Opin. Biotech. 45, 191-201 (2017), Biotechnology Advances 34, 31-41 (2016), Inorganic Chemistry 53, 1925-1942 (2014), Curr. Opin. Chem. Eng. 4, 79-87 (2014), in “DNA Nanotechnology: From Structure to Function”, Springer-Verlag Berlin Heidelberg; pp. 227-305 (2013), Chemical Reviews 109, 1948-1998 (2009)
I. Selection and Characterization of Novel DNAzymes and Aptamers
In fundamental science, designing selective sensors based on a single class of molecules for a broad range of targets remains a significant challenge. Most processes for sensor development proceed on a trial and error basis where successes in designing agents for one target can be difficult to translate into successes in designing agents for other targets. To meet these challenges, we have used in vitro selection or SELEX to obtain DNAzymes, a class of metalloenzymes that use DNA molecules exclusively for catalysis, and aptamers, a class of nucleic acids that rival antibodies in binding targets of choice strongly and specifically. In addition, by using counter selection techniques, we can improve the selectivity for our target of interest against other similar targets.

In the process of selecting new functional DNA, we strive to understand structural and chemical features of nucleic acid oligomers that are responsible for high selectivity and enzyme-like reaction mechanisms by using a suite of biochemical and spectroscopic methods (e.g. UV-vis, EPR, XAS), as well as x-ray crystallography, to improve the rational design of functional DNA.
Biochemistry 57, 1517-1522 (2018), Inorg. Chim. Acta 452, 12-24 (2016), Adv. Mater. 26, 7849-7872 (2014), Journal of the American Chemical Society 131, 5506-5515 (2009), ChemBioChem 10, 486-492 (2009), Nat Chem Biol 3, 763-768 (2007), Journal of the American Chemical Society 129, 6896-6902 (2007), Biochemistry 42, 7152-7161 (2003), Journal of the American Chemical Society 124, 15208-15216 (2002)

II. Design of DNAzyme and Aptamer-Based Sensors for in vitro Detection for Metal Ions and Small Molecule Targets
We are developing highly sensitive and selective sensors for metal ions and other small molecule targets, such as environmental contaminants, unsafe adulterants in foods, biomarkers for early disease detection, and therapeutic drug monitoring in point-of-care diagnostics. By conjugating the DNAzymes and aptamers obtained from in vitro selection with either a fluorophore and quencher, gold nanoparticles, or gadolinium/supermagnetic iron oxide nanoparticles, we have developed new classes of fluorescent, colorimetric, and MRI agents, respectively, for detecting metal ions and a wide range of other targets with high sensitivity (down to 14 pM) and selectivity (> 1 million fold selectivity).

III. Design of DNAzyme and Aptamer-Based Imaging Agents for Biological Systems

While metal ions and metabolites can be beneficial or toxic, the roles of these metabolites in human health is not fully understood. To offer deeper insight into their roles in biology, we are also developing imaging agents to study the distribution and the functional mechanisms of metal ions and small molecules in living cells and in vivo. These sensors can help fill the major gap in biomedical sciences where understanding of metabolomics lags significantly behind that of genomics and proteomics.

IV. Adapting the Widely Available, Low-cost, and Pocket-sized Glucose Meters for POC Diagnostics

In technological development, there are still significant barriers preventing the public from adopting new devices or technologies which are developed in academic laboratories. We are exploring ways to overcome these barriers by taking advantage of the wide availability and low cost of pocket-sized electrochemical devices, such as glucose meters, to detect many non-glucose targets, including vitamins (e.g. biotin), toxic metal ions (e.g. Pb2+), adulterants (e.g. melamine), toxins (e.g. aflatoxins), and disease markers (e.g. cancer).
V. Multimodal Theranostic Agents for Biomedical Applications
We are developing multimodal theranostic reagents that use aptamers as targeting agents and liposomes, which can encapsulate fluorescent, PET, and MRI imaging agents, as well as therapeutic drugs, for both detection and treatment of disease.

VI. Sensing and Imaging in Plant Biology
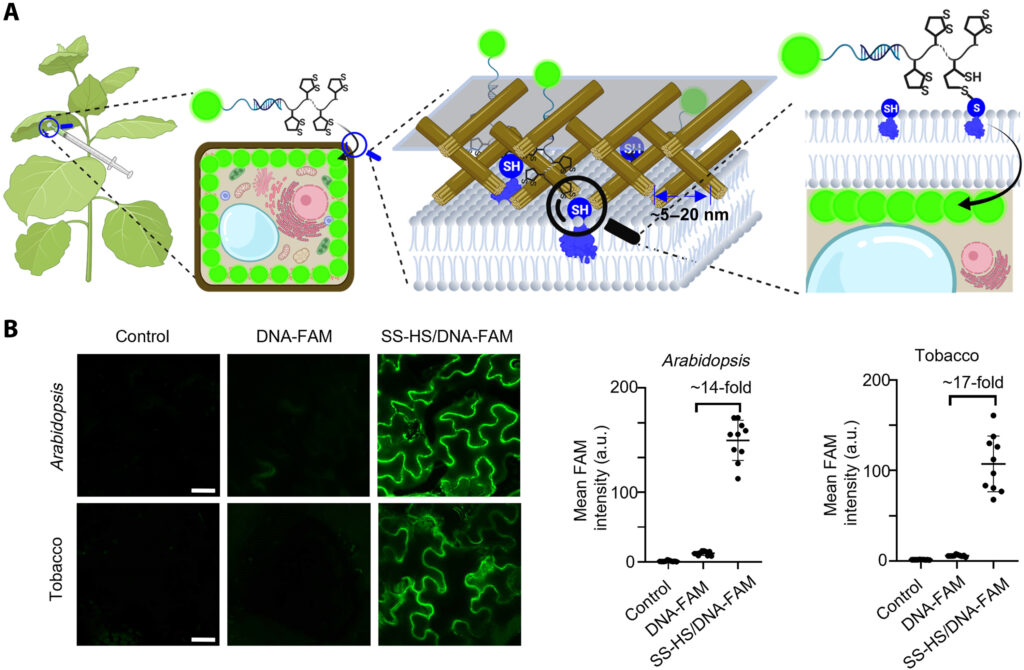
Having succeeded in sensing and imaging in mammalian cells, zebra fish and mice, we are developing methods to allow DNAzyme and aptamers to be delivered into plants for sensing and imaging plant biology. As the first step in this direction, we need to develop novel ways to delivery the DNAzyme/aptamers into plants, because the plant cell walls are quite different from those of mammalian cells and difficult to deliver DNA into plant cells efficiently. We have developed a thio-mediated approach to allow efficient delivery of DNA aptamers into plant cells.
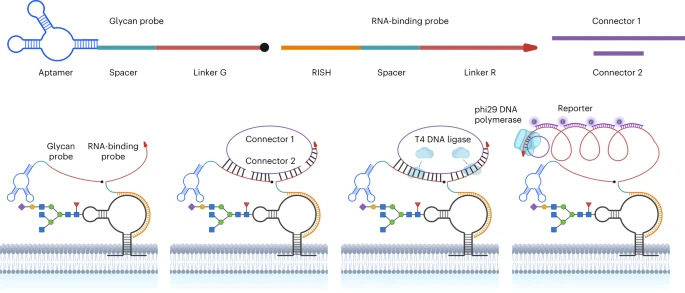
VII. Spatial Imaging of GlycoRNAs
GlycoRNAs were recently discovered as a new class of glycosylated biomolecules (Cell 184, 3109-3124 (2021)). In contrast to the widely studied proteoglycans, glycoproteins, and glycosphingolipids, detailed information on the glycoRNAs, especially their spatial distributions and expression levels on the surface of living cells, remains to be understood. Such information is critical to understand the biological functions of glycoRNAs and their roles in different diseases. To obtain the information, we have developed a novel imaging method called Sialic Acid Aptamer and RNA in situ Hybridization-mediated Proximity Ligation Assay (ARPLA) to visualize glycoRNAs on cell surfaces. We have applied ARPLA to reveal spatial distributions of glycoRNAs on the cell surface and discovered their colocalization with lipid rafts. We also observed the intracellular trafficking of glycoRNAs through SNARE protein-mediated secretory exocytosis. We further applied ARPLA to investigate the glycoRNA abundances in the breast cancer models and identified a glycoRNA downregulation during breast cancer progression and metastasis. This finding indicates that the surface glycoRNA has an inverse correlation to the tumor malignance and tumor metastasis. To demonstrate broad and diverse applicability of our ARPLA method, we have also applied the method to visualize glycoRNA molecules in immune cell models and revealed its reduction during immune cell differentiation and accrescence during pro-inflammatory responses. Finally, we demonstrated that glycoRNA can strengthen the interaction between monocyte/macrophage and vessel endothelial cells, suggesting its role in inflammatory responses.