Biosynthetic Inorganic Chemistry
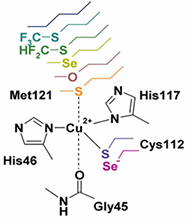
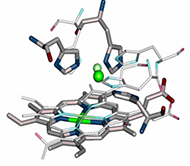
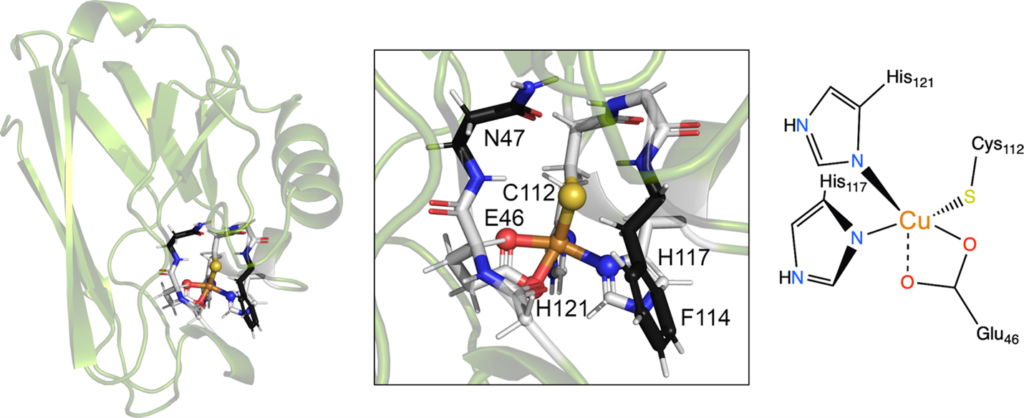
Metalloenzymes are important to biology because they catalyze essential and often complex processes in life including cellular respiration, photosynthesis, and nitrogen fixation. They are impressive to chemists because they do so under mild conditions and with high efficiency and selectivity. This has inspired us to design artificial enzymes that mimic or even surpass the abilities of native enzymes. To gain insight on how metalloenzymes work and design enzymes with novel functions, we take a “bottom-up” approach of engineering metal centers in small, stable, and well-characterized proteins. Using protein scaffolds instead of small organic molecules as ligands has many advantages, including faster production with higher yields and a rigid structure that allows us to probe the roles of non-covalent interactions in the secondary coordination sphere in controlling the activity and selectivity of the metalloenzymes.
Using this biosynthetic approach, we focus on designing environmentally benign catalysts with applications in renewable energy generation and small molecule activation or transformation. For example, we have designed metalloproteins with tunable redox potentials and studying their applications in many chemical and biological processes from efficient electron transfer (ET) agents in photosynthesis and respiration to catalysis in S-nitrosylation, water oxidation and N2 fixation. In addition, we have designed biosynthetic models of heteronuclear metalloenzymes such as heme-copper oxidases (HCOs), efficient oxygen reduction enzymes, nitric oxide reductases (NORs), which are involved in the nitrogen cycle, sulfite reductase, which is involved in sulfur cycle, and manganese peroxidases, which are involved in biomass conversion. Finally, we are introducing unnatural amino acids and non-native cofactors, including many inorganic and organometallic catalysts, to make them water-soluble, asymmetric catalysts for applications such as synthesizing chiral intermediates in pharmaceutical drugs.
| Approaches | Biomimetic Modeling | Protein Design |
|---|---|---|
| State of the arts | Using small organic molecules as ligands | Rational design using computer programs |
| Significant challenges | It is difficult to make biomimetics that are both structural and functional models, especially for complex metal-binding sites such as heteronuclear metallozymes. It is difficult to incorporate site-specific non-covalent interactions around the metal-binding sites. | It is much more difficult to design metalloproteins, as the metal-binding sites are not as well defined as non-metalloproteins. The activities (e.g., Kcat and turnovers) are often much lower than those of native enzymes. |
| Our hypotheses | Design and synthesis of the primary coordination sphere is often not enough; the non-covalent secondary coordination interactions around the metal-binding sites (e.g., hydrophobicity and hydrogen-bonding network, often involving site- specific water) play critical roles in both conferring and fine-tuning the enzymatic activities. | |
| Our major contributions | Designed metalloproteins that are both structural and functional models of native enzymes, by demonstrating site-specific incorporation of non-covalent secondary coordination sphere interactions around the metal-binding sites. The resulting metalloproteins often have high activities/turnovers, some of them with properties beyond natural range. The process has revealed hidden structural features responsible the high activities. |
|
| Some examples | 1. Employed unnatural amino acids to allow the blue copper center in Azurin to cover the physiological range of electron potential. 2. Engineered a heme [4Fe-4S] enzyme that catalyzes sulfite reduction like the native enzyme using modeling and rational design. 3. A structural and functional nitric oxide reductase (NOR) in myoglobin before NOR structure was available. 4. Demonstrated that the presence of non-covalent interactions play critical roles in conferring high activities. |
|
For in-depth reviews of our research, please see our recent publications in
Chem Rev, Comprehensive Coordination Chemistry, Protein Engineering: Tools and Applications, Acc. Chem. Res., Curr. Opin. Chem. Biol, BBA-Bioenergetics, Nature, Inorganic Chemistry, and Angewandte Chemie.