Deeper Understanding of S-Nitrosylation
Nitric oxide (NO) plays extensive roles in biology. At millimolar concentrations, macrophages use NO as an immune defense agent to combat invading pathogens. Meanwhile, at lower concentrations, NO is a critical signaling molecule, which largely occurs through nitrosylation/denitrosylation of cysteine, a reversible post-translational protein modification referred to as S-nitrosylation (SNO). As a result, NO (dys)regulation and processing has been linked to septic shock, cancer initiation, neurodegeneration, and cardiovascular diseases.
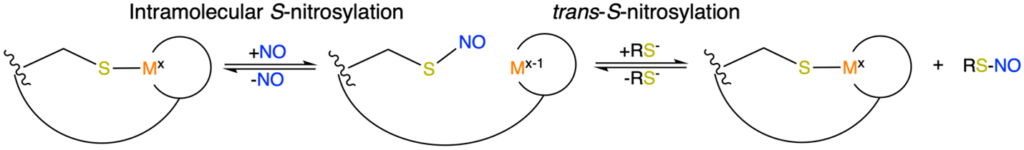
Figure 1. Schematic processes of intramolecular and trans-SNO. R represents small molecules, predominately glutathione in biologicial systems.
The SNO signaling pathway commonly involves a combination Cu and Fe-containing metalloenzymes and low molecular weight thiols such as glutathione and coenzyme A. Moreover, proteomic methods have identified thousands of SNO-modified proteins, highlighting the potential roles of less well characterized metalloenzymes in NO regulation. Therefore, understanding the fundamental, translatable principles of efficient metal-mediated SNO are critical to both to provide insight into NO regulation, and for the further development of effective therapeutics.
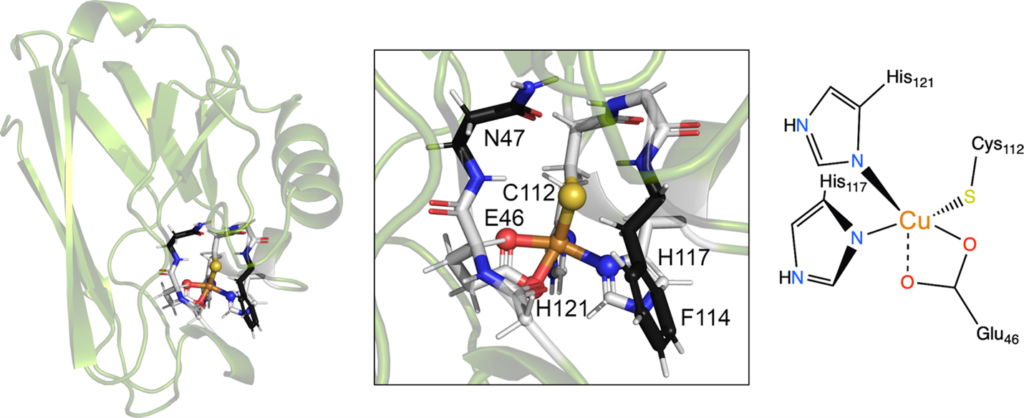
Figure 2. The reversible SNO artificial metalloenzyme, M121H/H46E azurin. The secondary coordination sphere residues N47 and F114 neighboring the Cu-SC112 site are shown (center), and a schematic of the primary coordination sphere is provided on the right.
To this end, we have developed Cu and Fe-containing non-heme artificial metalloenzymes capable of interacting with NO, both to perform reversible intramolecular S-nitrosylation, as well as bind NO at the metal center. We are actively investigating how the metal coordination environment influences the SNO and reverse de-SNO processes. In particular, we are exploring:
1.Using the primary and secondary coordination sphere to tune the equilibrium between NO bound/unbound states (ΔG°)
2.How protein environment can be used to minimize observed rate limiting barrier (ΔG‡)
3.Developing metalloproteins to promote trans-SNO processes with small molecules and other proteins.
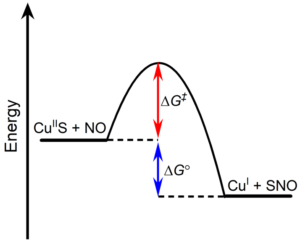
Figure 3. Target barriers to optimizing reversible SNO.